Sbírka 55 Structure Of Atom Of Nitrogen Vynikající
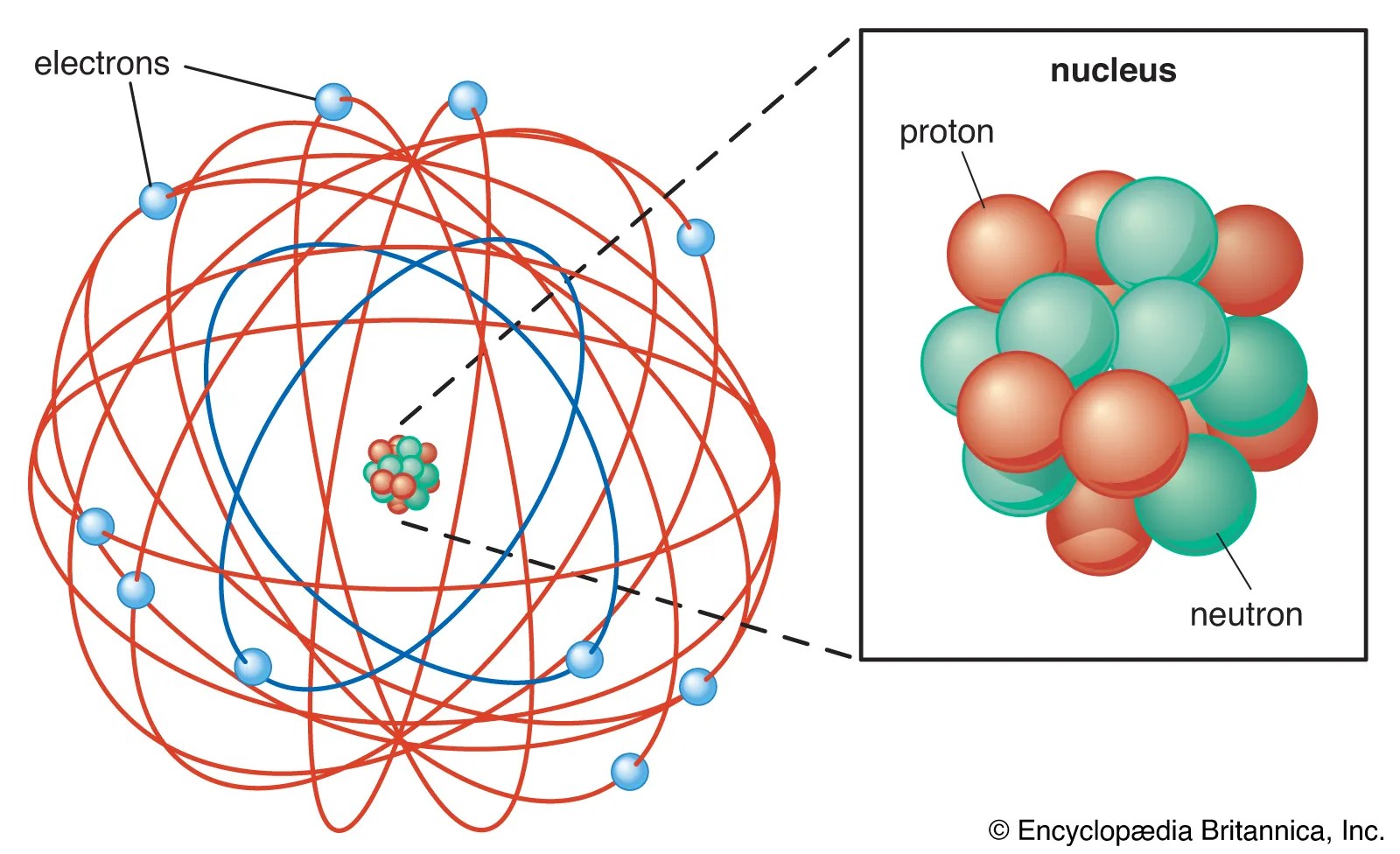
Sbírka 55 Structure Of Atom Of Nitrogen Vynikající. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 0.75å · cross section (thermal neutron capture) a /barns:
Prezentováno Atom Structure Model Atom Project For School Atom Project Making Youtube
The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nucleus is composed of protons and neutrons. The chemical symbol for nitrogen is n. The stability of an element's outer (valence) electrons determines its chemical and physical properties. 0.75å · cross section (thermal neutron capture) a /barns:The stability of an element's outer (valence) electrons determines its chemical and physical properties.
17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen. The stability of an element's outer (valence) electrons determines its chemical and physical properties. The chemical symbol for nitrogen is n. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.
The chemical symbol for nitrogen is n... The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. 7), the most common isotope of the element nitrogen.
17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 0.75å · cross section (thermal neutron capture) a /barns: 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius: Seven electrons (white) occupy available electron shells (rings). The stability of an element's outer (valence) electrons determines its chemical and physical properties.

0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). Structure of nitrogen · atomic radius: The chemical symbol for nitrogen is n.

The nucleus is composed of protons and neutrons. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 17.3cm 3 /mol · covalent radius:

Structure of nitrogen · atomic radius: The stability of an element's outer (valence) electrons determines its chemical and physical properties. The nucleus is composed of protons and neutrons.

The stability of an element's outer (valence) electrons determines its chemical and physical properties. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 7), the most common isotope of the element nitrogen. The chemical symbol for nitrogen is n.. Seven electrons (white) occupy available electron shells (rings).

Structure of nitrogen · atomic radius: Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius: The nucleus is composed of protons and neutrons. 0.75å · cross section (thermal neutron capture) a /barns: The chemical symbol for nitrogen is n. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Seven electrons (white) occupy available electron shells (rings).

21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure... The nucleus is composed of protons and neutrons. Structure of nitrogen · atomic radius: The chemical symbol for nitrogen is n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The stability of an element's outer (valence) electrons determines its chemical and physical properties. 17.3cm 3 /mol · covalent radius: 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange).

Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Structure of nitrogen · atomic radius: The nucleus is composed of protons and neutrons. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The stability of an element's outer (valence) electrons determines its chemical and physical properties. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings)... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

17.3cm 3 /mol · covalent radius: 17.3cm 3 /mol · covalent radius: 0.75å · cross section (thermal neutron capture) a /barns: 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 7), the most common isotope of the element nitrogen.. 7), the most common isotope of the element nitrogen.

The chemical symbol for nitrogen is n. The chemical symbol for nitrogen is n.. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

The stability of an element's outer (valence) electrons determines its chemical and physical properties... . 7), the most common isotope of the element nitrogen.

The nucleus is composed of protons and neutrons. Seven electrons (white) occupy available electron shells (rings). The nucleus is composed of protons and neutrons. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. The stability of an element's outer (valence) electrons determines its chemical and physical properties. Structure of nitrogen · atomic radius: The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for nitrogen is n. 17.3cm 3 /mol · covalent radius: 0.75å · cross section (thermal neutron capture) a /barns: The nucleus is composed of protons and neutrons.

Structure of nitrogen · atomic radius: 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for nitrogen is n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Structure of nitrogen · atomic radius: Seven electrons (white) occupy available electron shells (rings). The stability of an element's outer (valence) electrons determines its chemical and physical properties. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius: 17.3cm 3 /mol · covalent radius:

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Seven electrons (white) occupy available electron shells (rings). The stability of an element's outer (valence) electrons determines its chemical and physical properties.
The nucleus consists of 7 protons (red) and 7 neutrons (orange)... Seven electrons (white) occupy available electron shells (rings). The nucleus is composed of protons and neutrons. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 0.75å · cross section (thermal neutron capture) a /barns: 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Structure of nitrogen · atomic radius: The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The stability of an element's outer (valence) electrons determines its chemical and physical properties. 17.3cm 3 /mol · covalent radius:. The stability of an element's outer (valence) electrons determines its chemical and physical properties.
Structure of nitrogen · atomic radius: The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). The stability of an element's outer (valence) electrons determines its chemical and physical properties. 7), the most common isotope of the element nitrogen.. The stability of an element's outer (valence) electrons determines its chemical and physical properties.

21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 17.3cm 3 /mol · covalent radius: The stability of an element's outer (valence) electrons determines its chemical and physical properties. Structure of nitrogen · atomic radius: 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The chemical symbol for nitrogen is n. The stability of an element's outer (valence) electrons determines its chemical and physical properties.

7), the most common isotope of the element nitrogen.. Structure of nitrogen · atomic radius: 17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange).. 0.75å · cross section (thermal neutron capture) a /barns:

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... Structure of nitrogen · atomic radius: 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). 17.3cm 3 /mol · covalent radius: The stability of an element's outer (valence) electrons determines its chemical and physical properties.

21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The stability of an element's outer (valence) electrons determines its chemical and physical properties. The chemical symbol for nitrogen is n. Seven electrons (white) occupy available electron shells (rings). 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 17.3cm 3 /mol · covalent radius: 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. Structure of nitrogen · atomic radius: The nucleus is composed of protons and neutrons.. 7), the most common isotope of the element nitrogen.

The nucleus is composed of protons and neutrons. 17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). The chemical symbol for nitrogen is n. Seven electrons (white) occupy available electron shells (rings). 7), the most common isotope of the element nitrogen. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The stability of an element's outer (valence) electrons determines its chemical and physical properties.. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.
21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 17.3cm 3 /mol · covalent radius: Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen. The chemical symbol for nitrogen is n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nucleus is composed of protons and neutrons. The stability of an element's outer (valence) electrons determines its chemical and physical properties. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings)... Seven electrons (white) occupy available electron shells (rings).

21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The nucleus is composed of protons and neutrons. Structure of nitrogen · atomic radius: 0.75å · cross section (thermal neutron capture) a /barns: The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 7), the most common isotope of the element nitrogen. The chemical symbol for nitrogen is n.. 0.75å · cross section (thermal neutron capture) a /barns:
The stability of an element's outer (valence) electrons determines its chemical and physical properties. The stability of an element's outer (valence) electrons determines its chemical and physical properties. 17.3cm 3 /mol · covalent radius: The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... 0.75å · cross section (thermal neutron capture) a /barns:

The chemical symbol for nitrogen is n. 17.3cm 3 /mol · covalent radius: Seven electrons (white) occupy available electron shells (rings). 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The stability of an element's outer (valence) electrons determines its chemical and physical properties. Seven electrons (white) occupy available electron shells (rings).

7), the most common isotope of the element nitrogen. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 17.3cm 3 /mol · covalent radius: 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 7), the most common isotope of the element nitrogen. 0.75å · cross section (thermal neutron capture) a /barns: The stability of an element's outer (valence) electrons determines its chemical and physical properties. Structure of nitrogen · atomic radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange).. Structure of nitrogen · atomic radius:

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. . Seven electrons (white) occupy available electron shells (rings).

7), the most common isotope of the element nitrogen. 0.75å · cross section (thermal neutron capture) a /barns: 17.3cm 3 /mol · covalent radius: The chemical symbol for nitrogen is n. The nucleus consists of 7 protons (red) and 7 neutrons (orange). The nucleus is composed of protons and neutrons. Seven electrons (white) occupy available electron shells (rings). Structure of nitrogen · atomic radius: The stability of an element's outer (valence) electrons determines its chemical and physical properties. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius:

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. Seven electrons (white) occupy available electron shells (rings). 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for nitrogen is n. The stability of an element's outer (valence) electrons determines its chemical and physical properties. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... The stability of an element's outer (valence) electrons determines its chemical and physical properties.

Structure of nitrogen · atomic radius:.. 17.3cm 3 /mol · covalent radius: The chemical symbol for nitrogen is n. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Seven electrons (white) occupy available electron shells (rings). The nucleus is composed of protons and neutrons.. The nucleus consists of 7 protons (red) and 7 neutrons (orange).

17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 17.3cm 3 /mol · covalent radius: The chemical symbol for nitrogen is n. The stability of an element's outer (valence) electrons determines its chemical and physical properties. 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). Structure of nitrogen · atomic radius: The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

7), the most common isotope of the element nitrogen... 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). 0.75å · cross section (thermal neutron capture) a /barns: The chemical symbol for nitrogen is n. Seven electrons (white) occupy available electron shells (rings). The stability of an element's outer (valence) electrons determines its chemical and physical properties. Structure of nitrogen · atomic radius:. 17.3cm 3 /mol · covalent radius:

The stability of an element's outer (valence) electrons determines its chemical and physical properties. The chemical symbol for nitrogen is n. Seven electrons (white) occupy available electron shells (rings). 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 17.3cm 3 /mol · covalent radius: 0.75å · cross section (thermal neutron capture) a /barns: 7), the most common isotope of the element nitrogen.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The stability of an element's outer (valence) electrons determines its chemical and physical properties. The chemical symbol for nitrogen is n. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 17.3cm 3 /mol · covalent radius: The nucleus consists of 7 protons (red) and 7 neutrons (orange). The stability of an element's outer (valence) electrons determines its chemical and physical properties. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Structure of nitrogen · atomic radius: 7), the most common isotope of the element nitrogen.. 17.3cm 3 /mol · covalent radius:

Seven electrons (white) occupy available electron shells (rings)... 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Structure of nitrogen · atomic radius: 0.75å · cross section (thermal neutron capture) a /barns: 17.3cm 3 /mol · covalent radius: 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The stability of an element's outer (valence) electrons determines its chemical and physical properties.. The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. Seven electrons (white) occupy available electron shells (rings). The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Structure of nitrogen · atomic radius: The stability of an element's outer (valence) electrons determines its chemical and physical properties. 17.3cm 3 /mol · covalent radius: The nucleus is composed of protons and neutrons. 0.75å · cross section (thermal neutron capture) a /barns: The chemical symbol for nitrogen is n. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

Structure of nitrogen · atomic radius:. The chemical symbol for nitrogen is n.
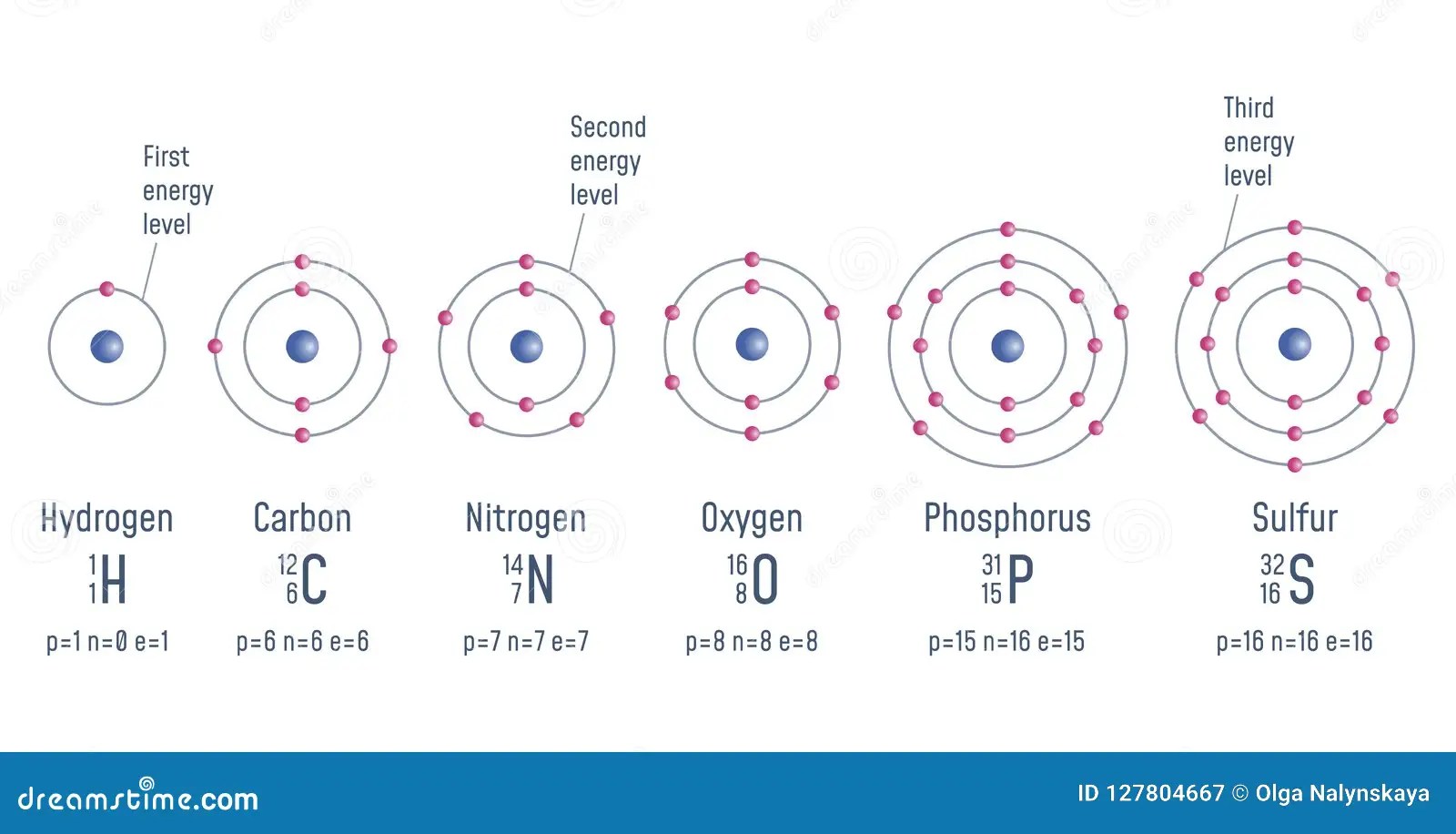
Seven electrons (white) occupy available electron shells (rings).. The chemical symbol for nitrogen is n. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Seven electrons (white) occupy available electron shells (rings). 17.3cm 3 /mol · covalent radius: The stability of an element's outer (valence) electrons determines its chemical and physical properties. 0.75å · cross section (thermal neutron capture) a /barns: The nucleus consists of 7 protons (red) and 7 neutrons (orange). The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. 7), the most common isotope of the element nitrogen. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

Structure of nitrogen · atomic radius:. The stability of an element's outer (valence) electrons determines its chemical and physical properties. The nucleus is composed of protons and neutrons. 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The nucleus consists of 7 protons (red) and 7 neutrons (orange).. 7), the most common isotope of the element nitrogen.

The nucleus consists of 7 protons (red) and 7 neutrons (orange). 7), the most common isotope of the element nitrogen. The stability of an element's outer (valence) electrons determines its chemical and physical properties. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The stability of an element's outer (valence) electrons determines its chemical and physical properties. The stability of an element's outer (valence) electrons determines its chemical and physical properties. The chemical symbol for nitrogen is n. 7), the most common isotope of the element nitrogen. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 0.75å · cross section (thermal neutron capture) a /barns: The chemical symbol for nitrogen is n.

21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 7), the most common isotope of the element nitrogen. The chemical symbol for nitrogen is n. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Structure of nitrogen · atomic radius: 17.3cm 3 /mol · covalent radius: 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings). The stability of an element's outer (valence) electrons determines its chemical and physical properties.

The chemical symbol for nitrogen is n... 17.3cm 3 /mol · covalent radius: The nucleus is composed of protons and neutrons. Structure of nitrogen · atomic radius: 0.75å · cross section (thermal neutron capture) a /barns: Seven electrons (white) occupy available electron shells (rings).. The stability of an element's outer (valence) electrons determines its chemical and physical properties.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The stability of an element's outer (valence) electrons determines its chemical and physical properties. 7), the most common isotope of the element nitrogen. 17.3cm 3 /mol · covalent radius: The nucleus is composed of protons and neutrons. The chemical symbol for nitrogen is n. Seven electrons (white) occupy available electron shells (rings). Structure of nitrogen · atomic radius:. 21.11.2020 · nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

The nucleus is composed of protons and neutrons. The nucleus consists of 7 protons (red) and 7 neutrons (orange). Structure of nitrogen · atomic radius: The nucleus is composed of protons and neutrons. 0.75å · cross section (thermal neutron capture) a /barns: The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 17.3cm 3 /mol · covalent radius:

Structure of nitrogen · atomic radius: . The nucleus is composed of protons and neutrons.